| Location: Home>News>Events |
| Prof. Sijin Liu's Group Report on New Progress in Metal Metabolism and Toxicity |
|
|
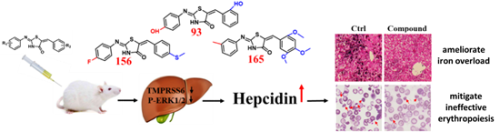
Recently, Prof. Sijin Liu (State Key Laboratory of Environmental Chemistry and Ecotoxicology, Research Center for Eco-Environmental Sciences, Chinese Academy of Sciences) and collaborators Prof. Bing Yan (School of Environmental Science and Engineering, Shandong University) and Prof. Tomas Ganz (Department of Medicine and Department of Pathology, David Geffen School of Medicine at University of California Los Angeles) achieved great progress in metal homeostasis and toxicological mechanisms. The findings have been published in Haematologica (Liu and Liu, et al. 2019,doi:10.3324/haematol.2018.209874), Advanced Science (Song, et al. 2018;5:1800866), American Journal of Hematology (Guo, et al. 2019;94:184-8) and Journal of Trace Elements in Medicine and Biology (Li, et al. 2019;52:232-8). Iron, a necessary element, plays an important role in many biological processes in normal physiology. Iron disorder caused by environmental pollutants or hereditary gene mutations results in a serial of diseases (e.g. anemia and iron overload). Hereditary hemochromatosis and β-thalassemia are typical iron overload diseases; however, there is no effective drug to treat these diseases. Current therapies such as iron depletion, blood transfusion and splenectomy can only reduce excess iron and have many limitations. Therefore, there is an urgent need for new drugs. Hepcidin, produced by hepatocytes, plays a central role in systemic iron homeostasis via binding to its receptor, ferroportin (FPN), through which it decreases iron efflux as well as intestinal iron absorption. Therefore, to enhance hepcidin production represents a promising strategy to prevent iron accumulation in iron overload disorders. In the current study, the authors screened the synthetic library of 210 thiazolidinones and identified three thiazolidinone compounds that effectively stimulated hepatic hepcidin expression. In a hemochromatosis mouse model with hemochromatosis deficiency, the three compounds prevented the development of iron overload. Moreover, these compounds also greatly ameliorated iron overload and mitigated ineffective erythropoiesis in β-thalassemic mice. Mechanistic studies revealed that TMPRSS6 reduction and diminished ERK1/2 phosphorylation were demonstrated to account for the enhanced expression of hepcidin (Figure 1). This work was published in Haematologica.
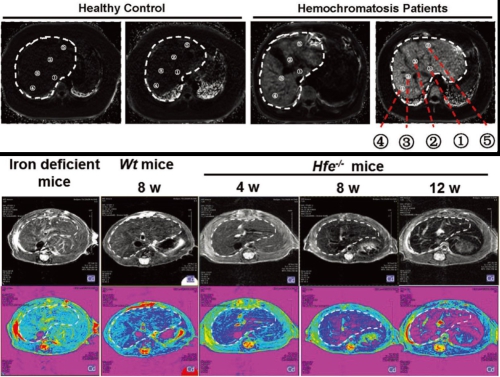
Figure 1. Thiazolidinones prevent iron overload and ameliorate ineffective hematopoiesis by increasing hepcidin expression Thus far, little is known about the profiles of iron deposition in different liver zones. In this study, uneven iron distribution in the liver is uncovered, showing the region with the highest iron concentration near the entrance site of the portal vein and hepatic artery in contrast to the sites with the lowest iron concentration close to the distal edge (Figure 2). Of note, the zones with greater iron accumulation appear to be more sensitive to iron changes. Moreover, mechanistic investigation manifests that these differential iron changes in liver zones are subjected to the regulation by the hepcidin–ferroportinaxis. The work was published in Advanced Science.
Figure 2. Significant regional differences of hepatic iron Additionally, this group revealed the hepcidin regulation to modulate iron availability during healthy and abnormal pregnancies. More importantly, they also synthesized a DFO derivative, DFCAF, and found that DFCAF displayed greater cellular permeability to chelate intracellular iron in cancer cells than DFO, posing more inhibition on cell growth and cellular motility or invasion. Importantly, DFCAF was uncovered to remarkably deplete cancer stem cells (CSCs), as characterized by the remarkable decrease of the CD44+/high/CD24-/low and ALDH+/high subpopulation. These research findings yield new insights into the mechanisms of iron metabolism under normal and disease conditions. The work was published in American Journal of Hematology and Journal of Trace Elements in Medicine and Biology. The above work was supported by grants from the National Natural Science Foundation of China and the Strategic Priority Research Program B of the Chinese Academy of Sciences.Links to related publications: http://www.haematologica.org/content/early/2019/02/15/haematol.2018.209874 https://onlinelibrary.wiley.com/doi/10.1002/advs.201800866 https://onlinelibrary.wiley.com/doi/full/10.1002/ajh.25341 https://www.ncbi.nlm.nih.gov/pubmed/30732888
State Key Laboratory of Environmental Chemistry and Ecotoxicology February 25, 2019
|